Introduction and Uses of Hydroxychloroquine
Hydroxychloroquine is a 4-amino-quinolone antiprotozoal and anti-inflammatory drug that is used in the treatment and management of malaria.
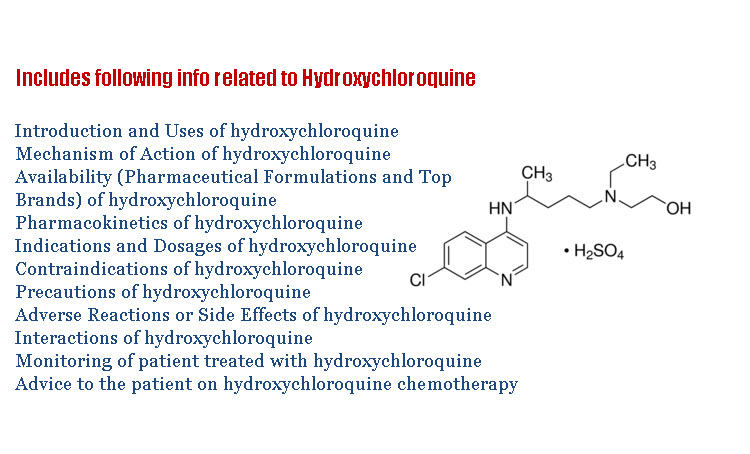
The chemical structure of 4-amino-quinolone i.e., hydroxychloroquine is as follows

The salt form of Hydroxychloroquine is hydroxychloroquine sulfate.
The other uses of hydroxychloroquine are as follows:
- Used in the management of Rheumatoid Arthritis (RA), thus it is considered as Antirhematoid drug particularly classified under Disease-Modifying Antirhematoid Drug (DMARD).
- Used in the management of Systemic Lupus Erythematosus (SLE).
- Used in the management of Porphyria Cutanea Tarda, Primary Sjogren Syndrome, Sarcoidosis, Chronic Q Fever, Noncardiac Organ Disease, and Postpartum with serologic evidence >12 months after delivery. All these uses of hydroxychloroquine are off-label.
Thus from the above information, hydroxychloroquine can be classified under
- Antiprotozoals
- Antimalarials
- Antirheumatoid (DMARDs)
- Anti-inflammatory
Mechanism of Action
Antimalarial Action
The exact mechanism is not known. However, it is thought to get concentrated in parasite acid vesicles thereby increasing the pH of the vesicles and interfere with the inhibition of protein synthesis and DNA replication thereby causing the parasite death.
Antirheumatoid Action (DMARD)
The exact mechanism is not known here too. However, they have been found to reduce monocyte IL-I, consequently inhibiting B Lymphocytes. Antigen processing may be interfered with. Lysosomal Stabilization and Free Radical Scavenging are the other proposed mechanisms. This hydroxychloroquine is found to induce remission in up-to 50% of patients of Rheumatoid Arthritis but should be taken for 3 to 6 months. The advantage is its toxicity is low however efficacy is also low. Bony erosions are not prevented by the use of this.
Availability (Pharmaceutical Formulations and Top Brands)
Available in tablet forms of 200mg, 300mg and 400mg.
200mg of hydroxychloroquine sulfate is equivalent to 155mg of hydroxychloroquine base…
Few common brand names of hydroxychloroquine are as follows:
| S. No. | Brand Name | Company |
| 1 | HCQ | Systemic Laboratories |
| 2 | HCQS | IPCA Laboratories |
| 3 | HQTOR | Torrent Vista |
| 4 | HYDROBET | Innovative |
| 5 | HYDROCAD | Cadila |
| 6 | HYDROQUIN | Sun Pharma |
| 7 | HYDROWIN | Icon Lifesciences |
| 8 | PAXOQUIN | Pax Healthcare |
| 9 | WINFLAM | Micro HC |
| 10 | ZY-Q | Zydus (Synovia) |
Indications and Dosages
Disease | Route of Administration | Adult | Pediatric |
|---|---|---|---|
| For Prophylaxis of Malaria (Chemoprophylaxis) | Oral | * 400mg once weekly on same day each week, begin 2 weeks before exposure. * If suppressive therapy is not begun prior to exposure, administer an initial loading dose of 800mg in 2 dividing doses, 6 hours apart. * Continue once weekly treatment for 8 weeks after leaving endemic area. Alternate Dosing: * 400mg once weekly on the same day each week, begin 1 to 2 week before travel to malarious area, continue therapy while in malarious area and for 4 weeks after leaving the area. | For Infants, Children and Adolescents: * 6.5mg/kg hydroxychloroquine sulfate once weekly on the same day each week; max dose: 400mg/dose hydroxychloroquine sulfate, begin 1 to 2 weeks before exposure. * Continue for atleast 4 weeks after after leaving endemic area. * If the initiation of chemoprophylaxis is delayed (i.e., 2 weeks of therapy not completed prior to exposure, initiate the therapy by doubling the initial dose (13mg/kg of hydroxychloroquine sulfate) and administering it in 2 divided doses 6 hours apart, max single dose: 400mg/dose hydroxychloroquine sulfate, continue for 8 weeks after leaving endemic area. |
| For Acute Attack of Malaria | Oral | 800mg initially, followed by 600mg at 6, 24 and 48 hours | * 13mg/kg/dose hydroxychloroquine sulfate (max initial dose: 800mg/dose hydroxychloroquine sulfate), followed by 6.5mg/kg hydroxychloroquine sulfate at 6, 24, and 48 hours after initial dose, max dose: 400mg/dose hydroxychloroquine sulfate |
| For Rheumatoid Arthritis | Oral | * 400mg daily as a single daily dose or in 2 divided doses, with or without concomitant non-biologic disease-modifying antirheumatic drugs. * Maintenance: 300mg daily (usually after 3 months of initial dosing). * Due to the risk of retinal toxicity, do not exceed a daily dose of 5mg/kg/day using actual body weight of 400mg | For Juvenile Rheumatoid Arthritis: 3-5mg/kg/day divided 1-2 times/day to a max of 400 mg/day, not to exceed 7 mg/kg/day. |
| For Porphyria Cutanea Tarda | Oral | * 100mg twice weekly. * Continue until plasma porphyrin levels are normal for at least one month. | - |
| For Primary Sjogren Syndrome (Extraglandular Manifestations) | Oral | * 200mg daily. * Due to the risk of retinal toxicity, do not exceed a daily dose of 5 mg/kg/day using actual body weight of 400mg. | - |
| For Chronic Q Fever | Oral | Endocarditis or Vascular Infection: * 200mg, Q8h in combination with doxycycline for ≥ 18 months. Noncardiac Organ Disease * 200mg, Q8h in combination with doxycycline. Postpartum with Serologic Evidence present ≥ 12 months after delivery: * 200mg, Q8h in combination with doxycycline for 12 months. | - |
| For Systemic Lupus Erythematosus | Oral | * 200-400mg daily as a single daily dose or in 2 divided doses. * Due to the risk of retinal toxicity, do not exceed a daily dose of 5 mg/kg/day using actual body weight of 400mg. | * 3-5mg/kg/day divided 1-2 times/day to a max of 400 mg/day, not to exceed 7 mg/kg/day. |