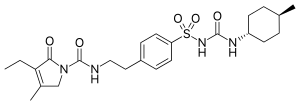
Glimepiride is an orally available medium-to-long-acting sulfonylurea antidiabetic drug. It is sometimes classified as either the first third-generation sulfonylurea, or as second generation.
Molecular Structure |
Class of Drug |
Oral hypoglycemic agent, second generation.
Mechanism of Action |
Stimulates release of insulin from pancreatic beta cells; decreases glucose production in liver; increases sensitivity of receptors for insulin, thereby promoting effectiveness of insulin.
Indications / Dosage / Route |
Routes of Administration: Oral only.
Condition: Diabetes, type II (non-insulin-dependent diabetes mellitus)
Dose: Adults: Initial: 1-2 mg/d with breakfast.
Maintenance: 1^1 mg once daily.
Adjustment of Dosage |
Kidney disease: None
Liver disease: None.
Elderly: Administer conservatively. Guidelines not available.
Pediatric: Not recommended.
| Onset of Action | Duration |
| 1-1.5 h | 10-16 h |
Food and Drug Interactions |
Food: No restriction. Patient should be told to eat regularly and not to skip meals. Sugar supply should be kept handy at all times. If stomach is upset Tums should be taken with meal. Dose is best administered before breakfast or, if taken twice a day, before the evening meal
Pregnancy: Category C.
Lactation: Probably appears in breast milk. Potentially toxic to infant. Avoid breastfeeding.
Contraindications: Hypersensitivity to glimepiride, diabetes complicated by ketoacidosis.
Warnings / Precautions |
> Current data suggests that there is an increased risk of cardiovascular mortality with oral hypoglycemic drugs.
> All sulfonylureas are capable of causing severe hypoglycemia. Patients should be educated concerning the signs and symptoms of hypoglycemia and how it can be prevented or reversed.
> When this drug is used to replace insulin, ininstruct patient to test urine for glucose and acetone at least 3 times a day and check results with treating physician.
> If the patient is experiencing stressful situations (surgery, infection, trauma), it may be necessary to administer insulin temporarily.
> A long-term prospective study performed by the University Group Diabetes Program showed that patients on tolbutamide (a sulfonylurea) had 2.5 times the risk of patients treated with diet alone.
Clinically Important Drug Interactions |
> Drugs that increase effects/toxicity of sulfonylureas: NSAIDs, P blockers, MAO inhibitors, cimetidine, glucocorticoids, alcohol (disulfiram effect).
> Drugs that decrease effects/toxicity of sulfonylureas: phenytoin, rifampin, cholestyramine.
Adverse Reactions |
> Common: GI upset.
> Serious: hypoglycemia, aplastic anemia, agranulocytosis, thrombocytopenia, hypersensitivity reaction, cholestatic jaundice.
Parameters to Monitor |
> Serum glucose, liver enzymes.
> Signs and symptoms of hyperglycemia
> Signs and symptoms of hypokalemia: muscle cramps or weakness, anorexia, thirst, hypoactive reflexes, polyuria, paresthesias, arrhythmias, dyspnea, confusion, paralytic ileus. If severe, hos¬pitalize patient and administer 50 mL of 50% glucose solution by rapid IV injection followed by 10% glucose infusion to maintain blood glucose at 90-180 mg/dL. Monitor patient for 24M8 hours for possible recurrence of hypoglycemia. For moderate hypoglycemia, administer Suit juices (1/2 cup orange juice), honey, sugar cubes (2), or com syrup. Follow this with milk or sandwich, which are sources of longer acting carbohydrate.
> Monitor therapeutic response carefully for the first 7 days after beginning treatment and for the first 3-5 days after transferring patient from insulin or another sulfonylurea. Continue monitoring to detect secondary failure after initial success; failure rate of oral hypoglycemic agent is 5-15% per year after 5 years of therapy.
> Determine glycosolated hemoglobin fraction (HbAlc or HbAj) levels 2—4 times a year. These are the best indices of glycemic con¬trol as they are indications of blood glucose over the previous 6-10 weeks.
> Possible causes of hypoglycemia.
> Signs and symptoms of bone marrow depression.
> Signs and symptoms of hepatotoxicity.
Advice to Patient |
> Do not undereat because skipping meals may result in loss of glucose control.
> Avoid even moderate amounts of alcohol, eg, more than 2 oz of 100-proof whiskey. The combination with the drug you are taking may result in a disulliram reaction: flushing, sweating, palpitation, nausea, vomiting, abdominal cramps.
> To minimize possible photosensitivity reactions, apply adequate sunscreen and use proper covering when exposed to strong sunlight.
> Carry identification card at all times describing disease, treatment regimen, name, address, and telephone number of treating physician.
> Carry hard candy or candy bar at all times.
Further Useful Info |
> In most cases, institute drug therapy only after a trial of 6-8 weeks of appropriate dietary control has not been successful in achieving satisfactory glycemic control. Begin drug at lowest dose and titrate upward ql-2wk.
> Patients at risk for hypoglycemia include those >60 years, alcoholics, malnourished patients, and those with severe or prolonged exertion, adrenal insufficiency, renal or hepatic dysfunction.
> Oral hypoglycemic agents should be prescribed only by physicians who are familiar with the proper criteria for patient selection and who know the risks versus benefits associated with these dmgs.